- Visibility 57 Views
- Downloads 12 Downloads
- DOI 10.18231/j.ijrimcr.2024.043
-
CrossMark
- Citation
Complicated community-acquired methicillin-resistant bacteremia leading to late-onset prosthetic joint infection: A breakthrough diagnosis with multiplex PCR
Introduction
Prosthetic joint infections (PJI) and other BJIs are associated with significant morbidity.[1] The diagnosis of PJI poses a significant challenge as at times the classical signs of infections may be absent and the radiology may also be not conclusive. Prior antibiotic therapy also may impact the gold-standard microbiological cultures. There is a need for diagnostic modalities which can provide a quick and accurate diagnosis.
Herein, we describe a case of SAB in a non-diabetic patient with bilateral TKR performed a year back where the BioFire® JI Panel established the diagnosis of late-onset PJI. To the best of our knowledge, this is the first case reported in India where the BioFire® JI Panel has been used to diagnose BJIs.
Case Report
A 73-year-old lady with a history sought medical attention in September 2022 for the sudden onset of progressively increasing fever with chills of 5 days' duration. This was associated with left-side lower back aches and weakness of the lower limbs. She subsequently also developed breathlessness, requiring oxygen support. She had undergone bilateral total knee replacement for osteoarthritis in 2021. She was initiated on ceftum and amikacin after admission. An infectious disease (ID) by the primary orthopedic consultation. On inquiry, the lady had skin problems with pruritis at the back, for which she had been seeking a dermatology opinion as well.
On examination, the patient was conscious and well-oriented to time, place, and person; febrile with 105°F with tachypnea. On systemic examination, bilateral crepitations were detected in the chest. There was mild tenderness at the right knee joint without any redness or apparent swelling.
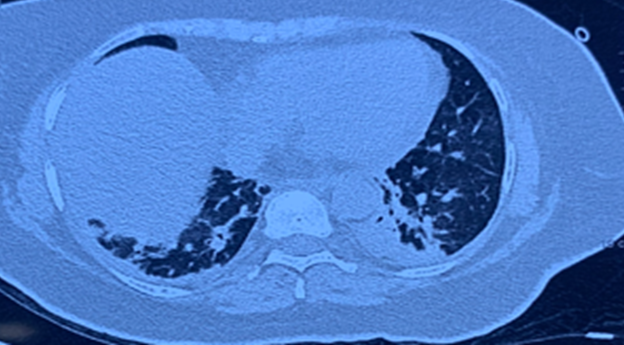
Her laboratory evaluation showed hemoglobin (Hb) 9.1 mg/dl, total leucocyte count (TLC) was 14,410 cells/cm3 with platelets 1.52 lakh/mm3, ESR 70 mm/hour, C-reactive protein (CRP) 378 mg/L, Procalcitonin (PCT) -3.93 ng/mL with creatinine was 1.9 mg/dl, and liver function tests were normal. Repeat blood cultures were advised, and antibiotics were modified to flucloxacillin and clindamycin with the clinical suspicion of SAB. Computed tomography (CT) chest revealed pneumonia ([Figure 1]).
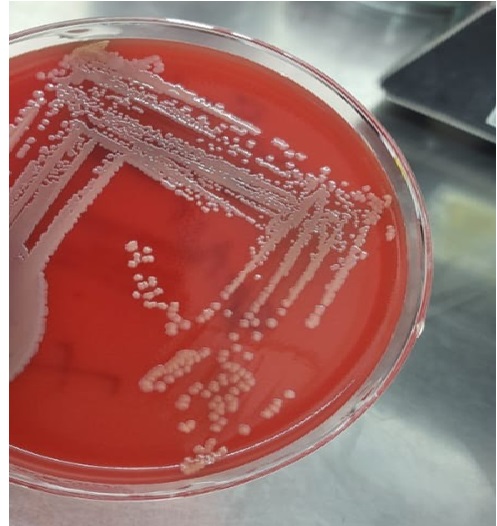
Blood cultures grew gram-positive cocci with time to positivity of 12 hours ([Figure 2]), later, identified as methicillin-resistant Staphylococcus aureus (MRSA) by VITEK 2 Compact (bioMérieux, Marcy-l'Étoile, France). The antibiotics were modified to teicoplanin, and clindamycin was continued.
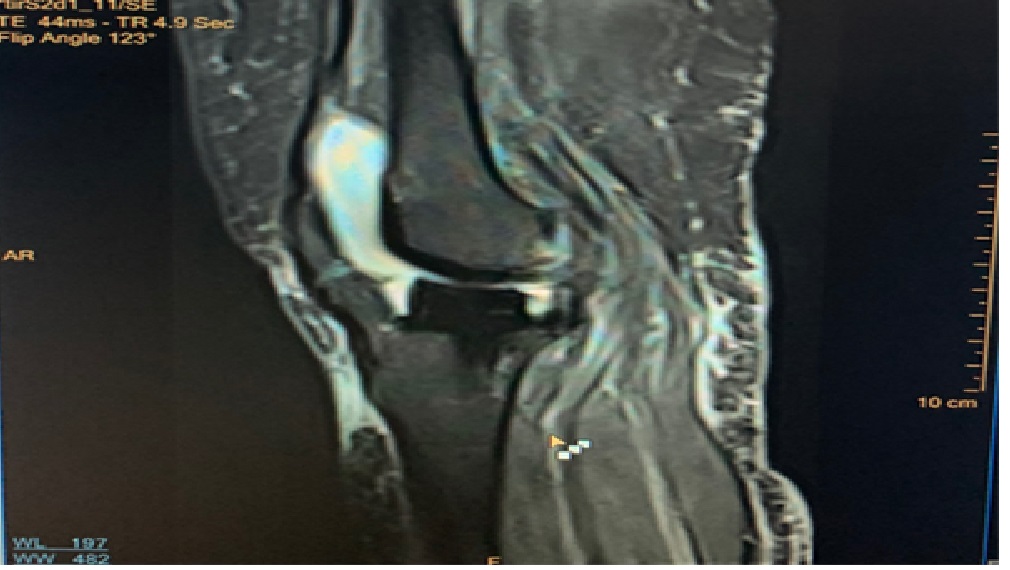
The patient clinically improved, and the repeat blood cultures after three days were sterile. However, the patient continued to have a leukocytosis with TLC of 39,600 cells/cm3 with mild tenderness persistent at the knee joints. The possibility of septic arthritis with hematogenous seeding of MRSA at the prosthetic knee joints was considered. However, the ultrasonography (USG)-guided aspiration of the knee joint was deferred at this point. As a part of work for leukocytosis - a repeat CT chest revealed an increase in the left-sided pleural effusion and pneumonia. Antibiotics were modified to daptomycin at 10 mg/kg in view of developing anemia after 6 days of teicoplanin. After 5 days of daptomycin and in view of persistent leukocytosis, an MRI of the bilateral knee joint was done, which revealed fluid collections with subcutaneous hyperintensities ([Figure 3]), but radiology was not conclusive.
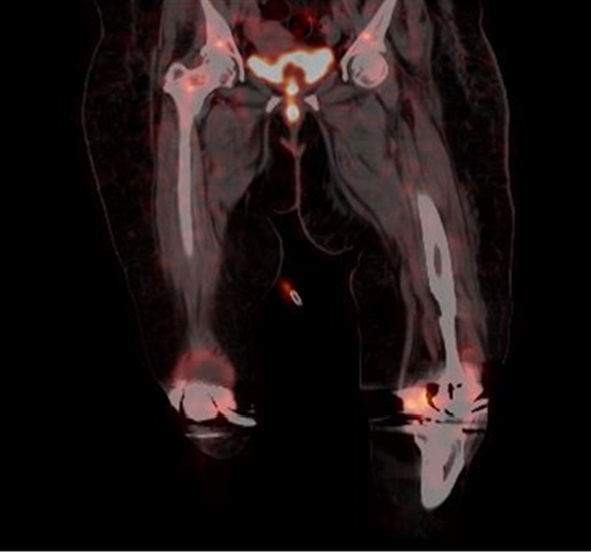
F18 fluorodeoxyglucose (FDG)-labeled leucocyte positron emission tomography (PET) scan, a promising diagnostic modality in the workup for metastatic infections for SAB, revealed bilateral knee effusion with increased labeled leucocyte uptake seen in the synovial lining suggestive of active infectious etiology ([Figure 4]).
A musculoskeletal USG-guided drainage of the fluid was done and sent for synovial fluid analysis, G stain, aerobic cultures, and the BioFire® JI Panel. The BioFire® JI Panel detected mecA/c and MREJ (MRSA) ([Figure 5]).
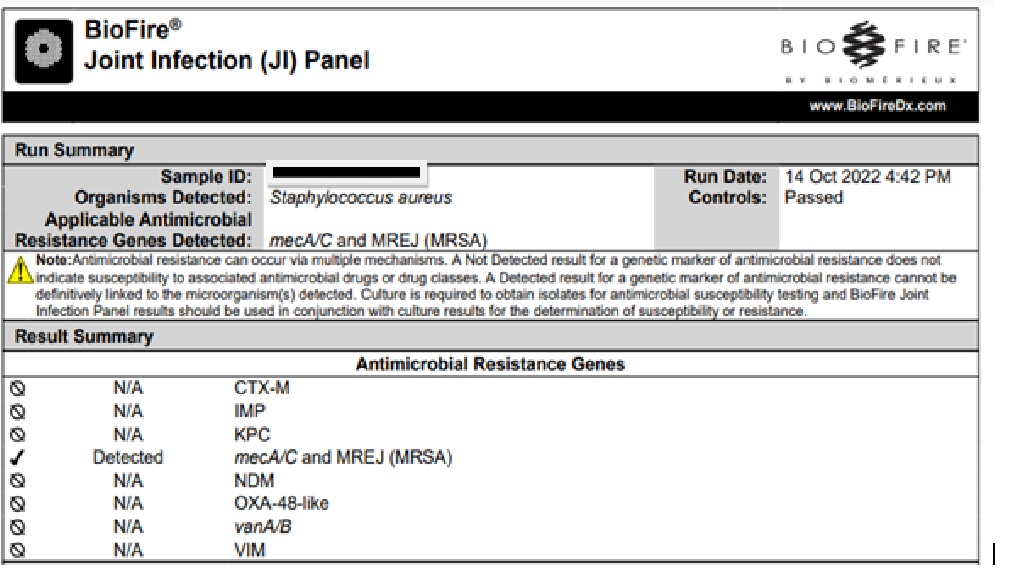
An immediate decision was taken by the orthopedic team for a surgical debridement of the knee joints with retention of the implants. Post debridement, the patient improved clinically as well, and the TLC was reduced to 15,000, with a decline in CRP. Synovial fluid analysis was available subsequently, which revealed a turbid fluid with 2.6 gm% proteins, total cell count (TCC) of 8289 cells/cm3 (59% neutrophils, 41% lymphocytes), glucose of less than 2 mg/dl. Later, the synovial fluid and the intraoperative cultures grew MRSA as well. The antibiotics were modified to oral trimethoprim – sulfamethoxazole with rifampicin. Later, due to intolerance and allergy, the patient was switched to oral alalevonadifloxacin owing to its efficacy, good bone penetration, tolerability and availability as an oral treatment option against MRSA.[2] The patient received antibiotic therapy for the late-onset PJI with CA-MRSA for six months including four and half months of oral alalevonadifloxacin. The patient has been doing well one and a half years after discontinuation of the antibiotics.
Discussion
Staphylococcus aureus bacteremia (SAB) poses significant clinical challenges, particularly in patients with prior orthopedic procedures such as total knee replacement (TKR). SAB is a heterogeneous infection with variable clinical manifestations and metastatic complications.[3]
The timely and accurate diagnosis of SAB is crucial for effective management, as delays can lead to inappropriate treatment and poor patient outcomes. Traditional microbiological methods, while foundational, often fall short in rapidly identifying pathogens, especially in cases of low-grade infections or when prior antibiotic therapy has been administered.[4] This highlights the need for advanced diagnostic tools, such as multiplex PCR assays, to enhance the management of SAB.
The BioFire® FilmArray JI Panel exemplifies the advancements in molecular diagnostics. This fully automated PCR-based assay provides rapid identification of multiple pathogens and antimicrobial resistance genes from a single sample within approximately one hour. In the context of SAB, the ability to quickly detect MRSA is particularly valuable. In our case, the BioFire® JI Panel detected MRSA with a significantly quicker turnaround time compared to traditional culture methods, which reported results 48 hours later. This rapid identification allowed for immediate surgical intervention with effective source control, adjustments in antibiotic therapy, facilitating more effective management of the infection and reducing the risk of complications associated with inappropriate antibiotic use.
The BioFire® JI Panel test is a comprehensive, fully automated ~1 hr sample-to-answer PCR-based molecular assay for the identification of 39 targets. This molecular assay detects 31 causative pathogens and 8 antimicrobial resistance genes (mecA/C in conjunction with the SCCmec right extremity junction [MREJ]), enterococcal resistance to vancomycin (vanA and vanB) and blaCTX-M, blaIMP, blaKPC, blaNDM, blaOXA-48-like, blaVIM) to guide surgical decision-making and appropriate antibiotics. Moreover, the ability of the BioFire JI Panel to detect polymicrobial infections enhances the understanding of the infection's complexity, which is particularly relevant in cases of bone and joint infections where multiple pathogens may be involved. This comprehensive detection capability allows for tailored surgical interventions and more effective management strategies.
A recent prospective study identified a cumulative positive detection rate of 100% (310/310) and expected negative detections of 99.3% (1,182/1,190) from 50 prototype BioFire JI Panel test runs. Unexpected detections of Streptococcus spp. (7/50) and Staphylococcus lugdunensis (1/50) were likely due to contaminants in the synovial fluid; Streptococcus spp. was confirmed by testing the synovial fluid in isolation.[5]
Another recent prospective study evaluated the performance of the BioFire JI Panel compared to culture as the standard of care (SoC). Out of the total 1544 synovial fluid specimens collected and tested with the BioFire JI Panel, majority of specimens were from knee joints (77.9%) and arthrocentesis (79.4%) was the most common collection method. Compared SoC, overall sensitivity was 90.2% and specificity was 99.8%.[6]
Despite the advantages of multiplex PCR, there are challenges to consider. The potential for false positives, as seen with unexpected detections of Streptococcus spp. in some studies, necessitates careful interpretation of results and consideration of clinical context.[5] Furthermore, the cost and availability of such advanced diagnostic tools may limit their widespread adoption in all healthcare settings.
In conclusion, the management of SAB, particularly in the context of emerging diagnostic technologies, requires a multifaceted approach.[7] Clinicians must balance the benefits of rapid and accurate diagnostics with the potential for false positives and false-negatives and the need for clinical correlation.[8] As advanced diagnostics can be expensive, healthcare providers should consider the cost-effectiveness of these tools in their specific settings, ensuring that they are accessible and beneficial for patient outcomes.
Source of Funding
None.
Conflict of Interest
None.
Acknowledgment
The authors are thankful to Dr Rajeev Soman for his support and advice on the manuscript.
References
- M Mcnally, R Sousa, M Wouthuyzen-Bakker, AF Chen, A Soriano, HC Vogely. The EBJIS definition of periprosthetic joint infection. Bone Joint J 2021. [Google Scholar]
- SS Bhagwat, M Nandanwar, A Kansagara, A Patel, S Takalkar, R Chavan. Levonadifloxacin, a novel broad-spectrum anti-MRSA benzoquinolizine quinolone agent: Review of current evidence. Drug Des Devel Ther 2019. [Google Scholar]
- IJ Kouijzer, VG Fowler Jr, Fowler, JT Oever. Redefining Staphylococcus aureus bacteremia: A structured approach guiding diagnostic and therapeutic management. J Infect 2023. [Google Scholar]
- MM Kheir, TL Tan, N Shohat, C Foltz, J Parvizi. Routine diagnostic tests for periprosthetic joint infection demonstrate a high false-negative rate and are influenced by the infecting organism. J Bone Joint Surg Am 2018. [Google Scholar]
- M Cronin, TK Fadgen, L Ogden, JP Green, SA Thatcher, RC Young. 640. Development of a laboratory verification protocol for concurrent detection of bacterial, fungal, and antimicrobial resistance genes in a multiplex syndromic joint infection pane. Open Forum Infect Dis 2021. [Google Scholar]
- C Graue, BH Schmitt, A Waggoner, F Laurent, L Abad, T Bauer. 322. Evaluation of the BioFire® Bone and Joint Infection (BJI) Panel for the detection of microorganisms and antimicrobial resistance genes in synovial fluid specimens. Open Forum Infect Dis 2020. [Google Scholar]
- LA Mermel, M Allon. Management of Staphylococcus aureus bacteremia: A clinical practice guideline. Clin Infect Dis 2022. [Google Scholar]
- S Pascual, B Noble, N Ahmad-Saeed, C Aldridge, S Ambretti, S Amit. Potential value of a rapid syndromic multiplex PCR for the diagnosis of native and prosthetic joint infections: A real-world evidence study. J Bone Jt Infect 2024. [Google Scholar]
How to Cite This Article
Vancouver
Gupta N, Gupta A. Complicated community-acquired methicillin-resistant bacteremia leading to late-onset prosthetic joint infection: A breakthrough diagnosis with multiplex PCR [Internet]. Int J Recent Innov Med Clin Res. 2025 [cited 2025 Sep 03];6(1):26-29. Available from: https://doi.org/10.18231/j.ijrimcr.2024.043
APA
Gupta, N., Gupta, A. (2025). Complicated community-acquired methicillin-resistant bacteremia leading to late-onset prosthetic joint infection: A breakthrough diagnosis with multiplex PCR. Int J Recent Innov Med Clin Res, 6(1), 26-29. https://doi.org/10.18231/j.ijrimcr.2024.043
MLA
Gupta, Neha, Gupta, Aarti. "Complicated community-acquired methicillin-resistant bacteremia leading to late-onset prosthetic joint infection: A breakthrough diagnosis with multiplex PCR." Int J Recent Innov Med Clin Res, vol. 6, no. 1, 2025, pp. 26-29. https://doi.org/10.18231/j.ijrimcr.2024.043
Chicago
Gupta, N., Gupta, A.. "Complicated community-acquired methicillin-resistant bacteremia leading to late-onset prosthetic joint infection: A breakthrough diagnosis with multiplex PCR." Int J Recent Innov Med Clin Res 6, no. 1 (2025): 26-29. https://doi.org/10.18231/j.ijrimcr.2024.043
